Related Resources: Electrical Design Engineering
Fuel Cell Technology Handbook
Electrical, Instrumentation, Electronic Design and Engineering
Battery Design, Engineering, Technology
Fuel Cell Technology Handbook
Mark C. Williams
National Energy Technology Laboratory
Strategic Center for Natural Gas
427 pages
Premium Membership Minimum Required to view Document/Book
Open: Fuel Cell Technology Handbook
Preface
Fuel cells are one of the cleanest and most efficient technologies for generating electricity. Since there is no combustion, there are none of the pollutants commonly produced by boilers and furnaces. For systems designed to consume hydrogen directly, the only products are electricity, water and heat. Fuel cells are an important technology for a potentially wide variety of applications including on-site electric power for households and commercial buildings; supplemental or auxiliary power to support car, truck and aircraft systems; power for personal, mass and commercial transportation; and the modular addition by utilities of new power generation closely tailored to meet growth in power consumption. These applications will be in a large number of industries worldwide.
In this Seventh Edition of the Fuel Cell Handbook, we have discussed the Solid State Energy Conversion Alliance Program (SECA) activities. In addition, individual fuel cell technologies and other supporting materials have been updated. Finally, an updated index assists the reader in locating specific information quickly.
It is an important task that NETL undertakes to provide you with this handbook. We realize it is an important educational and informational tool for a wide audience. We welcome suggestions to improve the handbook.
TOC
1. TECHNOLOGY OVERVIEW. 1-1
1.1 INTRODUCTION. 1-1
1.2 UNIT CELLS 1-2
1.2.1 Basic Structure 1-2
1.2.2 Critical Functions of Cell Components.. 1-3
1.3 FUEL CELL STACKING.. 1-4
1.3.1 Planar-Bipolar Stacking . 1-4
1.3.2 Stacks with Tubular Cells . 1-5
1.4 FUEL CELL SYSTEMS. 1-5
1.5 FUEL CELL TYPES 1-7
1.5.1 Polymer Electrolyte Fuel Cell (PEFC) 1-9
1.5.2 Alkaline Fuel Cell (AFC) 1-10
1.5.3 Phosphoric Acid Fuel Cell (PAFC) 1-10
1.5.4 Molten Carbonate Fuel Cell (MCFC) .. 1-11
1.5.5 Solid Oxide Fuel Cell (SOFC) . 1-12
1.6 CHARACTERISTICS 1-12
1.7 ADVANTAGES/DISADVANTAGES.. 1-14
1.8 APPLICATIONS, DEMONSTRATIONS, AND STATUS .. 1-15
1.8.1 Stationary Electric Power 1-15
1.8.2 Distributed Generation . 1-20
1.8.3 Vehicle Motive Power.. 1-22
1.8.4 Space and Other Closed Environment Power .. 1-23
1.8.5 Auxiliary Power Systems 1-23
1.8.6 Derivative Applications 1-32
1.9 REFERENCES 1-32
2. FUEL CELL PERFORMANCE.. 2-1
2.1 THE ROLE OF GIBBS FREE ENERGY AND NERNST POTENTIAL. 2-1
2.2 IDEAL PERFORMANCE .. 2-4
2.3 CELL ENERGY BALANCE. 2-7
2.4 CELL EFFICIENCY 2-7
2.5 ACTUAL PERFORMANCE 2-10
2.6 FUEL CELL PERFORMANCE VARIABLES 2-18
2.7 MATHEMATICAL MODELS 2-24
2.7.1 Value-in-Use Models 2-26
2.7.2 Application Models 2-27
2.7.3 Thermodynamic System Models. 2-27
2.7.4 3-D Cell / Stack Models .. 2-29
2.7.5 1-D Cell Models.. 2-31
2.7.6 Electrode Models. 2-32
2.8 REFERENCES 2-33
3. POLYMER ELECTROLYTE FUEL CELLS 3-1
3.1 CELL COMPONENTS 3-1
3.1.1 State-of-the-Art Components .. 3-2
3.1.2 Component Development 3-11
3.2 PERFORMANCE .. 3-14
3.3 PEFC SYSTEMS.. 3-16
3.3.1 Direct Hydrogen PEFC Systems . 3-16
3.3.2 Reformer-Based PEFC Systems.. 3-17
3.3.3 Direct Methanol Fuel Cell Systems .. 3-19
3.4 PEFC APPLICATIONS.. 3-21
3.4.1 Transportation Applications.. 3-21
3.4.2 Stationary Applications 3-22
3.5 REFERENCES 3-22
4. ALKALINE FUEL CELL 4-1
4.1 CELL COMPONENTS 4-5
4.1.1 State-of-the-Art Components .. 4-5
4.1.2 Development Components 4-6
4.2 PERFORMANCE . 4-7
4.2.1 Effect of Pressure .. 4-8
4.2.2 Effect of Temperature . 4-9
4.2.3 Effect of Impurities 4-11
4.2.4 Effects of Current Density.. 4-12
4.2.5 Effects of Cell Life. 4-14
4.3 SUMMARY OF EQUATIONS FOR AFC.. 4-14
4.4 REFERENCES 4-16
5. PHOSPHORIC ACID FUEL CELL . 5-1
5.1 CELL COMPONENTS 5-2
5.1.1 State-of-the-Art Components .. 5-2
5.1.2 Development Components 5-6
5.2 PERFORMANCE .. 5-11
5.2.1 Effect of Pressure 5-12
5.2.2 Effect of Temperature .. 5-13
5.2.3 Effect of Reactant Gas Composition and Utilization 5-14
5.2.4 Effect of Impurities 5-16
5.2.5 Effects of Current Density.. 5-19
5.2.6 Effects of Cell Life. 5-20
5.3 SUMMARY OF EQUATIONS FOR PAFC 5-21
5.4 REFERENCES 5-22
6. MOLTEN CARBONATE FUEL CELL . 6-1
6.1 CELL COMPONENTS 6-4
6.1.1 State-of-the-Art Componments .. 6-4
6.1.2 Development Components 6-9
6.2 PERFORMANCE .. 6-13
6.2.1 Effect of Pressure 6-15
6.2.2 Effect of Temperature .. 6-19
6.2.3 Effect of Reactant Gas Composition and Utilization 6-21
6.2.4 Effect of Impurities 6-25
6.2.5 Effects of Current Density.. 6-30
6.2.6 Effects of Cell Life. 6-30
6.2.7 Internal Reforming . 6-30
6.3 SUMMARY OF EQUATIONS FOR MCFC.. 6-34
6.4 REFERENCES 6-38
7. SOLID OXIDE FUEL CELLS.. 7-1
7.1 CELL COMPONENTS 7-2
7.1.1 Electrolyte Materials 7-2
7.1.2 Anode Materials . 7-3
7.1.3 Cathode Materials . 7-5
7.1.4 Interconnect Materials. 7-6
7.1.5 Seal Materials.. 7-9
7.2 CELL AND STACK DESIGNS . 7-13
7.2.1 Tubular SOFC .. 7-13
7.2.1.1 Performance . 7-20
7.2.2 Planar SOFC.. 7-31
7.2.2.1 Single Cell Performance. 7-35
7.2.2.2 Stack Performance. 7-39
7.2.3 Stack Scale-Up. 7-41
7.3 SYSTEM CONSIDERATIONS .. 7-45
7.4 REFERENCES 7-45
8. FUEL CELL SYSTEMS 8-1
8.1 SYSTEM PROCESSES .. 8-2
8.1.1 Fuel Processing .. 8-2
8.2 POWER CONDITIONING.. 8-27
8.2.1 Introduction to Fuel Cell Power Conditioning Systems.. 8-28
8.2.2 Fuel Cell Power Conversion for Supplying a Dedicated Load [2,3,4]. 8-29
8.2.3 Fuel Cell Power Conversion for Supplying Backup Power to a Load
Connected to a Local Utility . 8-34
8.2.4 Fuel Cell Power Conversion for Supplying a Load Operating in Parallel
With the Local Utility (Utility Interactive) 8-37
8.2.5 Fuel Cell Power Conversion for Connecting Directly to the Local Utility 8-37
8.2.6 Power Conditioners for Automotive Fuel Cells .. 8-39
8.2.7 Power Conversion Architecture for a Fuel Cell Turbine Hybrid Interfaced
With a Local Utility 8-41
8.2.8 Fuel Cell Ripple Current . 8-43
8.2.9 System Issues: Power Conversion Cost and Size 8-44
8.2.10 REFERENCES (Sections 8.1 and 8.2) .. 8-45
8.3 SYSTEM OPTIMIZATION. 8-46
8.3.1 Pressure 8-46
8.3.2 Temperature .. 8-48
8.3.3 Utilization 8-49
8.3.4 Heat Recovery.. 8-50
8.3.5 Miscellaneous 8-51
8.3.6 Concluding Remarks on System Optimization 8-51
8.4 FUEL CELL SYSTEM DESIGNS. 8-52
8.4.1 Natural Gas Fueled PEFC System . 8-52
8.4.2 Natural Gas Fueled PAFC System . 8-53
8.4.3 Natural Gas Fueled Internally Reformed MCFC System 8-56
8.4.4 Natural Gas Fueled Pressurized SOFC System 8-58
8.4.5 Natural Gas Fueled Multi-Stage Solid State Power Plant System . 8-62
8.4.6 Coal Fueled SOFC System. 8-66
8.4.7 Power Generation by Combined Fuel Cell and Gas Turbine System .. 8-70
8.4.8 Heat and Fuel Recovery Cycles .. 8-70
8.5 FUEL CELL NETWORKS . 8-82
8.5.1 Molten Carbonate Fuel Cell Networks: Principles, Analysis and
Performance .. 8-82
8.5.2 MCFC Network 8-86
8.5.3 Recycle Scheme .. 8-86
8.5.4 Reactant Conditioning Between Stacks in Series 8-86
8.5.5 Higher Total Reactant Utilization .. 8-87
8.5.6 Disadvantages of MCFC Networks 8-88
8.5.7 Comparison of Performance.. 8-88
8.5.8 Conclusions 8-89
8.6 HYBRIDS 8-89
8.6.1 Technology. 8-89
8.6.2 Projects. 8-92
8.6.3 World’s First Hybrid Project. 8-93
8.6.4 Hybrid Electric Vehicles (HEV) . 8-93
8.7 FUEL CELL AUXILIARY POWER SYSTEMS 8-96
8.7.1 System Performance Requirements 8-97
8.7.2 Technology Status.. 8-98
8.7.3 System Configuration and Technology Issues . 8-99
8.7.4 System Cost Considerations 8-102
8.7.5 SOFC System Cost Structure . 8-103
8.7.6 Outlook and Conclusions . 8-104
8.8 REFERENCES. 8-104
9. SAMPLE CALCULATIONS. 9-1
9.1 UNIT OPERATIONS.. 9-1
9.1.1 Fuel Cell Calculations . 9-1
9.1.2 Fuel Processing Calculations 9-13
9.1.3 Power Conditioners 9-16
9.1.4 Others 9-16
9.2 SYSTEM ISSUES.. 9-16
9.2.1 Efficiency Calculations 9-17
9.2.2 Thermodynamic Considerations.. 9-19
9.3 SUPPORTING CALCULATIONS. 9-22
9.4 COST CALCULATIONS. 9-25
9.4.1 Cost of Electricity 9-25
9.4.2 Capital Cost Development . 9-26
9.5 COMMON CONVERSION FACTORS 9-27
9.6 AUTOMOTIVE DESIGN CALCULATIONS. 9-28
9.7 REFERENCES 9-29
10. APPENDIX . 10-1
10.1 EQUILIBRIUM CONSTANTS .. 10-1
10.2 CONTAMINANTS FROM COAL GASIFICATION. 10-2
10.3 SELECTED MAJOR FUEL CELL REFERENCES, 1993 TO PRESENT.. 10-4
10.4 LIST OF SYMBOLS.. 10-10
10.5 FUEL CELL RELATED CODES AND STANDARDS. 10-14
10.5.1 Introduction. 10-14
10.5.2 Organizations . 10-15
10.5.3 Codes & Standards.. 10-16
10.5.4 Codes and Standards for Fuel Cell Manufacturers.. 10-17
10.5.5 Codes and Standards for the Installation of Fuel Cells . 10-19
10.5.6 Codes and Standards for Fuel Cell Vehicles .. 10-19
10.5.7 Application Permits. 10-19
10.5.8 References 10-21
10.6 FUEL CELL FIELD SITE DATA.. 10-21
10.6.1 Worldwide Sites 10-21
10.6.2 DoD Field Sites . 10-24
10.6.3 IFC Field Units.. 10-24
10.6.4 FuelCell Energy. 10-24
10.6.5 Siemens Westinghouse.. 10-24
10.7 HYDROGEN 10-31
10.7.1 Introduction. 10-31
10.7.2 Hydrogen Production . 10-32
10.7.3 DOE’s Hydrogen Research . 10-34
10.7.4 Hydrogen Storage. 10-35
10.7.5 Barriers.. 10-36
10.8 THE OFFICE OF ENERGY EFFICIENCY AND RENEWABLE ENERGY WORK IN FUEL
CELLS .. 10-36
10.9 RARE EARTH MINERALS 10-38
List of Figures
viii
LIST OF FIGURES
Figure Title Page
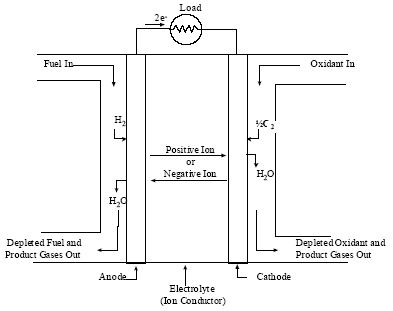
Figure 1-1 Schematic of an Individual Fuel Cell. 1-2
Figure 1-2 Expanded View of a Basic Fuel Cell Unit in a Fuel Cell Stack (1) 1-4
Figure 1-3 Fuel Cell Power Plant Major Processes . 1-7
Figure 1-4 Relative Emissions of PAFC Fuel Cell Power Plants Compared to Stringent
Los Angeles Basin Requirements . 1-13
Figure 1-5 PC-25 Fuel Cell.. 1-16
Figure 1-6 Combining the SOFC with a Gas Turbine Engine to Improve Efficiency .. 1-19
Figure 1-7 Overview of Fuel Cell Activities Aimed at APU Applications. 1-24
Figure 1-8 Overview of APU Applications . 1-24
Figure 1-9 Overview of typical system requirements. 1-25
Figure 1-10 Stage of development for fuel cells for APU applications .. 1-26
Figure 1-11 Overview of subsystems and components for SOFC and PEFC systems 1-28
Figure 1-12 Simplified process flow diagram of pre-reformer/SOFC system . 1-29
Figure 1-13 Multilevel system modeling approach 1-30
Figure 1-14 Projected Cost Structure of a 5kWnet APU SOFC System. .. 1-32
Figure 2-1 H2/O2 Fuel Cell Ideal Potential as a Function of Temperature. 2-5
Figure 2-2 Effect of fuel utilization on voltage efficiency and overall cell efficiency
for typical SOFC operating conditions (800 °C, 50% initial hydrogen Concentration).2-10
Figure 2-3 Ideal and Actual Fuel Cell Voltage/Current Characteristic . 2-11
Figure 2-4 Example of a Tafel Plot . 2-13
Figure 2-5 Example of impedance spectrum of anode-supported SOFC operated at
850 °C. 2-14
Figure 2-6 Contribution to Polarization of Anode and Cathode 2-17
Figure 2-7 Voltage/Power Relationship 2-19
Figure 2-8 The Variation in the Reversible Cell Voltage as a Function of Reactant
Utilization .2-23
Figure 2-9 Overview of Levels of Fuel Cell Models.. 2-26
Figure 2-10 Conours of Current Density on Electrolyte . 2-31
Figure 2-11 Typical Phenomena Considered in a 1-D Model (17) 2-32
Figure 2-12 Overview of types of electrode models (9).. 2-33
Figure 3-1 (a) Schematic of Representative PEFC (b) Single Cell Structure of
Representative PEFC .3-2
Figure 3-2 PEFC Schematic (4, 5).. 3-3
Figure 3-3 Polarization Curves for 3M 7 Layer MEA (12) 3-7
Figure 3-4 Endurance Test Results for Gore Primea 56 MEA at Three Current
Densities.3-10
Figure 3-5 Multi-Cell Stack Performance on Dow Membrane (9).. 3-12
Figure 3-6 Effect on PEFC Performance of Bleeding Oxygen into the Anode
Compartment (1)3-13
Figure 3-7 Evolutionary Changes in PEFCs Performance [(a) H2/O2, (b) H2/Air,
(c) Reformate Fuel/Air, (d) H2/unkown)] [24, 10, 12, , ] . 3-14
Figure 3-8 Influence of O2 Pressure on PEFC Performance (93°C, Electrode Loadings
of 2 mg/cm2 Pt, H2 Fuel at 3 Atmospheres) [(56) Figure 29, p. 49]. 3-15
Figure 3-9 Cell Performance with Carbon Monoxide in Reformed Fuel (56) .. 3-16
Figure 3-10 Typical Process Flow Diagram Showing Major Components of Direct
Hydrogen PEFC System 3-17
Figure 3-11 Schematic of Major Unit Operations Typical of Reformer-Based PEFC
Systems. .3-18
Figure 3-12 Comparison of State-of-the-Art Single Cell Direct Methanol Fuel Cell
Data (58) 3-21
Figure 4-1 Principles of Operation of H2/O2 Alkaline Fuel Cell, Immobilized
Electrolyte (8) 4-4
Figure 4-2 Principles of Operation of H2/Air Alkaline Fuel Cell, Circulating
Electrolyte (9) 4-4
Figure 4-3 Evolutionary Changes in the Performance of AFCs (8, 12, & 16) . 4-8
Figure 4-4 Reversible Voltage of the Hydrogen-Oxygen Cell (14) .. 4-9
Figure 4-5 Influence of Temperature on O2, (air) Reduction in 12 N KOH. . 4-10
Figure 4-6 Influence of Temperature on the AFC Cell Voltage 4-11
Figure 4-7 Degradation in AFC Electrode Potential with CO2 Containing and CO2
Free Air ..4-12
Figure 4-8 iR-Free Electrode Performance with O2 and Air in 9 N KOH at 55 to 60°C.
Catalyzed (0.5 mg Pt/cm2 Cathode, 0.5 mg Pt-Rh/cm2 Anode) Carbon-based
Porous Electrodes (22) 4-13
Figure 4-9 iR Free Electrode Performance with O2 and Air in 12N KOH at 65 °C 4-14
Figure 4-10 Reference for Alkaline Cell Performance. 4-15
Figure 5-1 Principles of Operation of Phosphoric Acid Fuel Cell (Courtesy of UTC
Fuel Cells)5-2
Figure 5-2 Improvement in the Performance of H2-Rich Fuel/Air PAFCs 5-6
Figure 5-3 Advanced Water-Cooled PAFC Performance (16). 5-8
Figure 5-4 Effect of Temperature: Ultra-High Surface Area Pt Catalyst. Fuel: H2,
H2 + 200 ppm H2S and Simulated Coal Gas (37) . 5-14
Figure 5-5 Polarization at Cathode (0.52 mg Pt/cm2) as a Function of O2 Utilization,
which is Increased by Decreasing the Flow Rate of the Oxidant at
Atmospheric Pressure 100 percent H3PO4, 191°C, 300 mA/cm2, 1 atm. (38) 5-15
Figure 5-6 Influence of CO and Fuel Gas Composition on the Performance of Pt
Anodes in 100 percent H3PO4 at 180°C. 10 percent Pt Supported on Vulcan
XC-72, 0.5 mg Pt/cm2. Dew Point, 57°. Curve 1, 100 percent H2; Curves
2-6, 70 percent H2 and CO2/CO Contents (mol percent) Specified (21) .. 5-18
Figure 5-7 Effect of H2S Concentration: Ultra-High Surface Area Pt Catalyst (37). 5-19
Figure 5-8 Reference Performances at 8.2 atm and Ambient Pressure. Cells from Full
Size Power Plant (16).. 5-22
Figure 6-1 Principles of Operation of Molten Carbonate Fuel Cells (FuelCell Energy). 6-2
Figure 6-2 Dynamic Equilibrium in Porous MCFC Cell Elements (Porous electrodes
are depicted with pores covered by a thin film of electrolyte) . 6-4
Figure 6-3 Progress in the Generic Performance of MCFCs on Reformate Gas and
Air (12, 13). 6-6
Figure 6-4 Effect of Oxidant Gas Composition on MCFC Cathode Performance at
650°C, (Curve 1, 12.6 percent O2/18.4 percent CO2/69.0 percent N2;
Curve 2, 33 percent O2/67 percent CO2) (49, Figure 3, Pg. 2711) .. 6-14
Figure 6-5 Voltage and Power Output of a 1.0/m2 19 cell MCFC Stack after 960 Hours
at 965 °C and 1 atm, Fuel Utilization, 75 percent (50).. 6-15
Figure 6-6 Influence of Cell Pressure on the Performance of a 70.5 cm2 MCFC at
650 °C (anode gas, not specified; cathode gases, 23.2 percent O2/3.2 percent
CO2/66.3 percent N2/7.3 percent H2O and 9.2 percent O2/18.2 percent
CO2/65.3 percent N2/7.3 percent H2O; 50 percent CO2, utilization at
215 mA/cm2) (53, Figure 4, Pg. 395) .. 6-18
Figure 6-7 Influence of Pressure on Voltage Gain (55) 6-19
Figure 6-8 Effect of CO2/O2 Ratio on Cathode Performance in an MCFC, Oxygen
Pressure is 0.15 atm (22, Figure 5-10, Pgs. 5-20).. 6-22
Figure 6-9 Influence of Reactant Gas Utilization on the Average Cell Voltage of an
MCFC Stack (67, Figure 4-21, Pgs. 4-24) 6-23
Figure 6-10 Dependence of Cell Voltage on Fuel Utilization (69) 6-25
Figure 6-11 Influence of 5 ppm H2S on the Performance of a Bench Scale MCFC
(10 cm x 10 cm) at 650 °C, Fuel Gas (10 percent H2/5 percent CO2/
10 percent H2O/75 percent He) at 25 percent H2 Utilization (78, Figure 4,
Pg. 443) ..6-29
Figure 6-12 IIR/DIR Operating Concept, Molten Carbonate Fuel Cell Design (29) 6-31
Figure 6-13 CH4 Conversion as a Function of Fuel Utilization in a DIR Fuel Cell
(MCFC at 650 ºC and 1 atm, steam/carbon ratio = 2.0, >99 percent methane
conversion achieved with fuel utilization > 65 percent (93) 6-33
Figure 6-14 Voltage Current Characteristics of a 3kW, Five Cell DIR Stack with
5,016 cm2 Cells Operating on 80/20 percent H2/CO2 and Methane (85).. 6-33
Figure 6-15 Performance Data of a 0.37m2 2 kW Internally Reformed MCFC Stack at
650 °C and 1 atm (13). 6-34
Figure 6-16 Average Cell Voltage of a 0.37m2 2 kW Internally Reformed MCFC Stack
at 650 °C and 1 atm. Fuel, 100 percent CH4, Oxidant, 12 percent CO2/9
percent O2/77 percent N2 . 6-35
Figure 6-17 Model Predicted and Constant Flow Polarization Data Comparison (98) 6-37
Figure 7-1 Electrolyte Conductivity as a Function of Temperature (4, 5, 6) 7-3
Figure 7-2 (a) Sulfur Tolerance of Ni-YSZ Anodes (16, 17) and (b) Relationship
between Fuel Sulfur and Anode Sulfur Concentration. 7-5
Figure 7-3 Impact of Chromia Poisoning on the Performance of Cells with Different
Electrolytes (From (21)) .. 7-6
Figure 7-4 Stability of Metal Oxides in Stainless Steels (26,27) 7-8
Figure 7-5 Impact of LSCM Contact Layer on Contact Resistance in Cell with Metal
Interconnect (from (28)). . 7-8
Figure 7-6 Possible Seal Types in a Planar SOFC (from (29)) . 7-10
Figure 7-7 Expansion of Typical Cell Components in a 10 cm x 10 cm Planar SOFC
with Ni-YSZ anode, YSZ Electrolyte, LSM Cathode, and Ferritic Steel
Interconnect..7-11
Figure 7-8 Structure of Mica and Mica-Glass Hybrid Seals and Performance of
Hybrid Seals (29) .. 7-13
Figure 7-9 Three Types of Tubular SOFC: (a) Conduction around the Tube (e.g.
Siemens Westinghouse and Toto (31)); (b) Conduction along the Tube
(e.g. Acumentrics (32)); (c) Segmented in Series (e.g. Mitsubishi Heavy
Industries, Rolls Royce (33,34)). .. 7-14
Figure 7-10 Cell Performance and Dimensions of Accumentrics Technology (32). 7-15
Figure 7-11 Schematic cross-section of cylindrical Siemens Westinghouse SOFC Tube. 7-16
Figure 7-12 Gas Manifold Design for a Tubular SOFC and Cell-to-Cell Connections in
a Tubular SOFC (41) .. 7-19
Figure 7-13 Performance Advantage of Sealless Planar (HPD5) over Conventional
Siemens Westinghouse Technology (42.). 7-21
Figure 7-14 Effect of Pressure on AES Cell Performance at 1,000 °C (2.2 cm diameter,
150 cm active length).. 7-22
Figure 7-15 Two-Cell Stack Performance with 67 percent H2 + 22 percent CO + 11
percent H2O/Air . 7-23
Figure 7-16 Two Cell Stack Performance with 97% H2 and 3% H2O/Air (43) .. 7-25
Figure 7-17 Cell Performance at 1,000 °C with Pure Oxygen (o) and Air (∆) Both at 25
percent Utilization (Fuel (67 percent H2/22 percent CO/11 percent H2O)
Utilization is 85 percent) 7-26
Figure 7-18 Influence of Gas Composition of the Theoretical Open-Circuit Potential
of SOFC at 1,000 °C 7-27
Figure 7-19 Variation in Cell Voltage as a Function of Fuel Utilization and Temperature
(Oxidant (o - Pure O2; ∆ - Air) Utilization is 25 percent. Current Density is
160 mA/cm2 at 800, 900 and 1,000 °C and 79 mA/cm2 at 700 °C). 7-28
Figure 7-20 SOFC Performance at 1,000 °C and 350 mA/cm2, 85 percent Fuel
Utilization and 25 percent Air Utilization (Fuel = Simulated Air-Blown
Coal Gas Containing 5,000 ppm NH3, 1 ppm HCl and 1 ppm H2S) .. 7-29
Figure 7-21 Voltage-Current Characteristics of an AES Cell (1.56 cm Diameter,
50 cm Active Length) . 7-30
Figure 7-22 Overview of Types of Planar SOFC: (a) Planar Anode-Supported SOFC
with Metal Interconnects(68); (b) Electrolyte-Supported Planar SOFC
Technology with Metal Interconnect (57,58,68); (c) Electrolyte-Supported
Design with “egg-crate” electrolyte shape and ceramic interconnect
(62,63,64,65)7-33
Figure 7-23 Representative State-of-the-Art Button Cell Performance of Anode-
Supported SOFC (1) .. 7-37
Figure 7-24 Single Cell Performance of LSGM Electrolyte (50 µm thick) .. 7-38
Figure 7-25 Effect of Oxidant Composition on a High Performance Anode-Supported
Cell7-39
Figure 7-26 Examples of State-of-the-Art Planar Anode-Supported SOFC Stacks and
Their Performance Characteristics (69,79,78) 7-40
Figure 7-27 Trend in Cell and Single-Cell-Stack Performance in Planar SOFC (69).. 7-41
Figure 7-28 Siemens Westinghouse 250 kW Tubular SOFC Installation (31) 7-42
Figure 7-29 Example of Window-Pane-Style Stack Scale-Up of Planar Anode-Supported
SOFC to 250 kW 7-43
Figure 8-1 A Rudimentary Fuel Cell Power System Schematic 8-1
Figure 8-2 Representative Fuel Processing Steps & Temperatures 8-3
Figure 8-3 “Well-To-Wheel” Efficiency for Various Vehicle Scenarios (9) 8-9
Figure 8-4 Carbon Deposition Mapping of Methane (CH4) 8-24
Figure 8-5 Carbon Deposition Mapping of Octane (C8H18) 8-24
Figure 8-6 Block diagram of a fuel cell power system.. 8-27
Figure 8-7a Typical fuel cell voltage / current characteristics . 8-28
Figure 8-7b Fuel cell power vs. current curve.. 8-28
Figure 8-8 Block diagram of a typical fuel cell powered unit for supplying a load
(120V/240V)8-30
Figure 8-9a Block diagram of the power conditioning unit with line frequency
transformer8-31
Figure 8-9b Circuit topology of the power conditioning unit with line frequency
transformer8-31
Figure 8-10a Block diagram of the power conditioning unit with high frequency isolation
transformer within the DC-DC converter stage . 8-32
Figure 8-10b Circuit topology of the power conditioning unit with high frequency
isolation transformer within the DC-DC converter stage . 8-32
Figure 8-11a Block diagram of the power conditioning unit with fewer power conversion
stages in series path of the power flow .. 8-33
Figure 8-11b Circuit topology of the power conditioning unit with fewer power
conversion stages in series path of the power flow.. 8-33
Figure 8-12 Fuel cell power conditioner control system for powering dedicated loads . 8-33
Figure 8-13 Diagram of a modular fuel cell power conversion unit for supplying backup
power to a load connected to a local utility [10,11]. 8-34
Figure 8-14 Modular power conditioning circuit topology employing two fuel cells to
supply a load via a line frequency isolation transformer [10,11] . 8-36
Figure 8-15 Modular power conditioning circuit topology employing two fuel cells
using a higher voltage (400V) dc-link [10,11] 8-36
Figure 8-16 Fuel cell supplying a load in parallel with the utility.. 8-37
Figure 8-17 Fuel cell power conditioner control system for supplying power to the
utility (utility interface).. 8-38
Figure 8-18 A typical fuel cell vehicle system [16] .. 8-39
Figure 8-19 Power conditioning unit for fuel cell hybrid vehicle .. 8-40
Figure 8-20 Fuel cell power conditioner control system [16] .. 8-40
Figure 8-21 Power conditioning unit for the 250kW fuel cell turbine hybrid system.. 8-41
Figure 8-22 Alternative power conditioning unit for the fuel cell turbine hybrid system
with shared dc-link [19]. 8-42
Figure 8-23 Possible medium voltage power conditioning topology for megawatt range
hybrid fuel cell systems [19] 8-43
Figure 8-24 Representative cost of power conditioning as a function of power and
dc-link voltage 8-44
Figure 8-25 Optimization Flexibility in a Fuel Cell Power System.. 8-47
Figure 8-26 Natural Gas Fueled PEFC Power Plant . 8-52
Figure 8-27 Natural Gas fueled PAFC Power System . 8-54
Figure 8-28 Natural Gas Fueled MCFC Power System .. 8-56
Figure 8-29 Schematic for a 4.5 MW Pressurized SOFC 8-58
Figure 8-30 Schematic for a 4 MW Solid State Fuel Cell System 8-63
Figure 8-31 Schematic for a 500 MW Class Coal Fueled Pressurized SOFC.. 8-66
Figure 8-32 Regenerative Brayton Cycle Fuel Cell Power System .. 8-71
Figure 8-33 Combined Brayton-Rankine Cycle Fuel Cell Power Generation System 8-74
Figure 8-34 Combined Brayton-Rankine Cycle Thermodynamics 8-75
Figure 8-35 T-Q Plot for Heat Recovery Steam Generator (Brayton-Rankine).. 8-76
Figure 8-36 Fuel Cell Rankine Cycle Arrangement.. 8-77
Figure 8-37 T-Q Plot of Heat Recovery from Hot Exhaust Gas . 8-78
Figure 8-38 MCFC System Designs.. 8-83
Figure 8-39 Stacks in Series Approach Reversibility 8-84
Figure 8-40 MCFC Network . 8-87
Figure 8-41 Estimated performance of Power Generation Systems.. 8-91
Figure 8-42 Diagram of a Proposed Siemens-Westinghouse Hybrid System.. 8-91
Figure 8-43 Overview of Fuel Cell Activities Aimed at APU Applications. 8-96
Figure 8-44 Overview of APU Applications . 8-96
Figure 8-45 Overview of typical system requirements. 8-97
Figure 8-46 Stage of development for fuel cells for APU applications .. 8-98
Figure 8-47 Overview of subsystems and components for SOFC and PEFC systems . 8-100
Figure 8-48 Simplified System process flow diagram of pre-reformer/SOFC system . 8-101
Figure 8-49 Multilevel system modeling approach. 8-102
Figure 8-50 Projected cost structure of a 5kWnet APU SOFC system. Gasoline fueled
POX reformer, Fuel cell operating at 300mW/cm2, 0.7 V, 90 percent fuel
utilization, 500,000 units per year production volume. .. 8-104
Figure 10-1 Equilibrium Constants (Partial Pressures in MPa) for (a) Water Gas Shift,
(b) Methane Formation, (c) Carbon Deposition (Boudouard Reaction), and
(d) Methane Decomposition (J.R. Rostrup-Nielsen, in Catalysis Science and
Technology, Edited by J.R. Anderson and M. Boudart, Springer-Verlag,
Berlin GDR, p.1, 1984.).. 10-2