Related Resources: Heat Transfer Engineering Design
Constant-Pressure Oxidation Propane in Air Excel Spreadsheet Calculator
NOTE:
- Refunds are not awarded after excel files have been downloaded - review your membership agreement for details.
- This excel spreadsheet may contain macros which will need to be enabled in your excel application, see web page: Enable macros in Downloaded excel files
- Units utilized within calculators are either SI or Imperial (some enable both SI and Imperial) and member (you) are responsible for conversions. Ensure that you verify units utilized in excel application meet your requirements before downloading.
Fluids Flow Engineering and Design
Heat Transfer Design and Engineering
Constant-Pressure Oxidation of Propane in Air Excel Spreadsheet Calculator
Note: Accessing this resource requires an active Premium (Annual) Membership with Engineers Edge
Download: Constant-Pressure Oxidation of Propane in Air Excel Spreadsheet Calculator
This spreadsheet models the constant-pressure oxidation of propane in air according to the 4-step mechanism of Hautmann et al., Combustion Science and Technology, vol. 25, pp. 219-235 (1981). The units have been converted from gram-mole, cm, s to kg-mole, m, s.
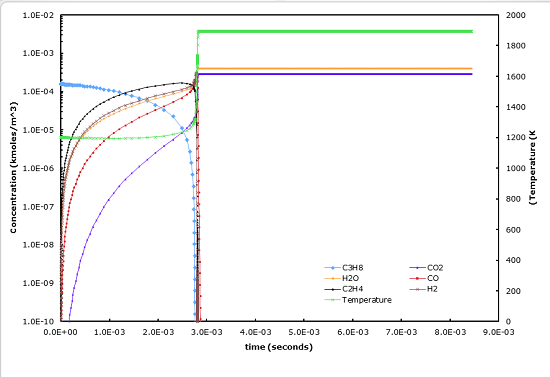
Notes:
1. Initial mole fractions of CO, CO2, H2O, C2H4, and H2 should not need to be changed either
2. dt_min and dt_max are the minimum and maximum time steps, should not need to be changed either
3. Same applies to minimum species concentration
4. The reaction rate expression for R2 has a negative exponent on the [C3H8] concentration,
and R4 has a negative exponent on [C2H4]. So the singularity as [C3H8] or [C2H4] -> 0
must be dealt with somehow; in this spreadsheet this is done simply by specifiying a minimum
[C3H8] or [C2H4] concentration in the reaction rate expression. This of course doesn't mean
that the actual concentrations can't go lower, just that the minimum value of concentration
used in the reaction rate equation doesn't go lower
5. Model includes
a. Variable molecular weight due to changing composition
b. T-dependent Cp (but Cp = Cp of N2 only is assumed, the effect of the other species on Cp has not been included yet)
This underestimates Cp and thus overestimates T, which may underestimate ignition time.
c. Thermal expansion due to heat release and molecular weight change
(which affects the moles/m^3 of the species, even if that species doesn't change mole fraction)
d. The enthalpies of all species and their effect on heat release & T
e. A way of determining the time step required (though it's crude and may be subject to further changes)
6. The calculations seem to be accurate. The atom balances are ok,
and there is pretty good agreement with Hautman's calculations (see 'Checks' sheet).
Also the sequence of reactions seems just fine, with CO -> CO2 being the last step.
Credit:
Paul Ronney
University of Southern California
Related: