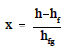Enthalpy Temperature (h-T) Diagram - Thermodynamics
Thermodynamics Directory | Heat Transfer Directory
Enthalpy Temperature (h-T) Diagram
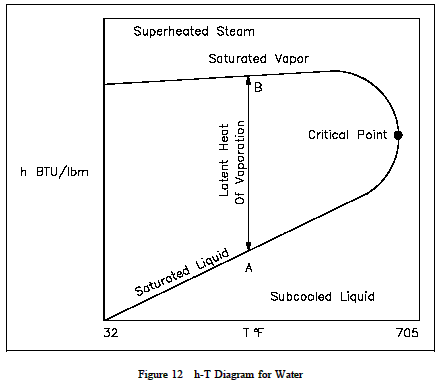
An Enthalpy Temperature h-T diagram exhibits the same features as on the previous property diagrams. Figure 12 is the h-T diagram for pure water. An h-T diagram can be constructed for any pure substance. As in the previous property diagrams, there are regions on the h-T diagram in which two phase sexist together. The region between the saturated liquid line and the saturated vapor line represents the area of two phases existing at the same time. The vertical distance between the two saturation lines represents the latent heat of vaporization. If pure water existed at point Aon the saturated liquid line and an amount of heat was added equal to the latent heat of vaporization, then the water would change phase from a saturated liquid to a saturated vapor(point B), while maintaining a constant temperature. As shown in Figure 12, operation outside the saturation lines results in a sub-cooled liquid or superheated steam.
The quality of the mixture at any point in the liquid-vapor region can be found using the same relationship as shown for the P-h diagram.