Fusion - Melting Change of Liquid State - Thermodynamics
Thermodynamics Directory | Heat Transfer Directory
Fusion - Melting Change of Liquid State Thermodynamics
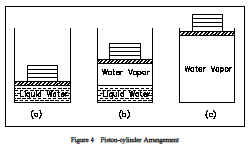
Consider one further experiment with the piston-cylinder arrangement of Figure 4. Suppose the cylinder contained 1 lbm of ice at 0F, 14.7 psia. When heat is transferred to the ice, the pressure remains constant, the specific volume increases slightly, and the temperature increases until it reaches 32F, at which point the ice melts while the temperature remains constant. In this state the ice is called a saturated solid. For most substances, the specific volume increases during this melting process, but for water the specific volume of the liquid is less than the specific volume of the solid. This causes ice to float on water. When all the ice is melted, any further heat transfer causes an increase in temperature of the liquid. The process of melting is also referred to as fusion. The heat added to melt ice into a liquid is called the latent heat of fusion.