Related Resources: thermodynamics
Introduction to Thermodynamics, Class 1
Thermodynamics Resources and Apps
Class 01 – Introduction to Thermodynamics
Objective(s):
At the completion of the lecture, students should:
1) Know their instructor.
2) Understand the class rules, grading scale, and expectations in the syllabus.
3) Understand when homeworks are assigned and due.
4) Understand homeworks are to follow the standard format.
5) Gain a general understanding of thermodynamics as a science applicable to engineering systems.
Methodology:
Lecture.
Reading:
Feynman Lectures on Physics Chapter 39 (Sections 39-1, 39-2, 39-4, 39-5) Audio Available!
Key Ideas:
Thermodynamics is the study of energy movement:
Energy can take many forms; in this course, we will study Work, Heat, and mass transport as ways to transfer energy.
Work is what we want. It’s the most useful way to transport energy. We want a piston to extend (or retract) or a shaft to spin. It’s characterized by consolidated, coordinated, coherent motion.
Heat is what we have to work with. It’s the least useful way to transport energy. It’s disordered and chaotic and takes time to be transported into or out of our system. But it’s conveniently cheap and abundant.
So, from the flow of heat, we extract some useful work, but also reject heat back into the environment as a form of pollution (i.e. heat energy that is so uselessly degraded it just gets thrown into the oceans, lakes, and atmosphere).
Where does the work go? Generating electricity. Pumping fluids. Lifting weights. Causing vehicular motion.
The total amount of energy, regardless of its form, going into the system will always equal the total amount either being stored inside of or leaving the system. This is the Conservation of Energy principle, the First Law of Thermodynamics.
Eventually, however, all that useful work finds a way to degrade into useless heat energy. This is arguably the most sacred (i.e. most disprovable) physical law – the Second Law of Thermodynamics.
Visualizing the macroscopic (use the stirling engines as demonstrative devices here):
Work is easy to see happen and easy to measure.
Pressure, expansion, compression, distance, force, rate of translation, rate of rotation, torque, mass, acceleration, etc.
Heat must be inferred and imagined.
We use temperature to infer an object’s energy content, implying the amount of heat it can absorb or shed. How jiggly is my reservoir of matter? Can I make it jigglier by allowing it to communicate with another reservoir? Jiggliness always goes from an area of more jiggliness to less jiggliness. Or, in smarter language, heat always flows from regions of higher temperature to regions of lower temperature.
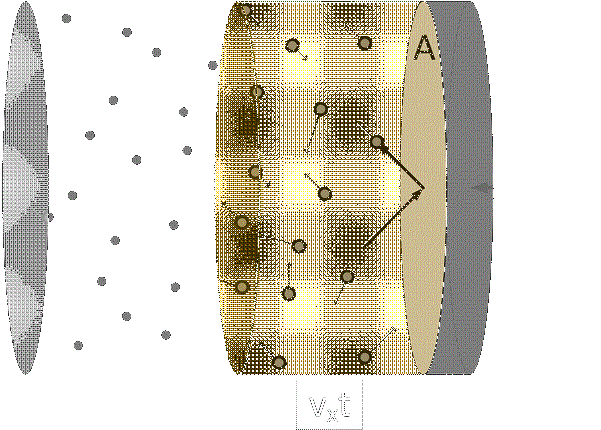
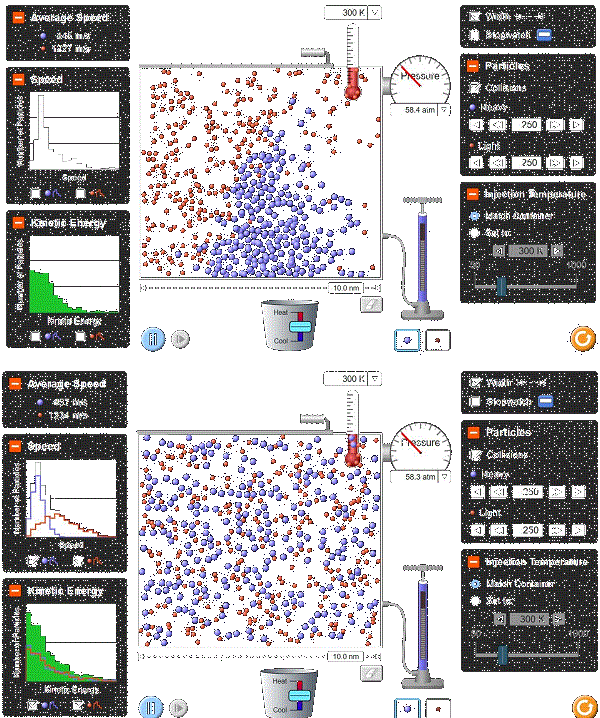
Visualizing the microscopic (use the ideal gas java app as a demonstrative device here):
Picturing the ballistic trajectories of molecules has proven to be the easiest way for me to understand thermodynamics.
This is the kinetic theory of gases, and we’ll come back to it in future lectures.
Suffice it to say, think of gas molecules as a bunch of tiny billiard or ping pong balls bouncing off each other and the walls of their container, and you might be able to visualize the idealized macroscopic processes this course will employ.
It’s hard to understand a machine by just looking at it from the outside. You might be able to measure its power output and rotational rate and such, but that doesn’t really tell you about its guts. By looking inside, you can see how the individual components culminate into the overall observed behavior.
Techniques for success in this course:
Engineering is difficult because it requires strong qualitative AND quantitative analysis skills.
Qualitative – applying your current knowledge of physical laws to imagine how a certain arrangement, system, etc. will play out. Basically, educated guessing and visualizing on how the answer might look.
Quantitative – using the numbers and units available to us to flesh out our qualitative intuition.
Combining the quantitative answer with the qualitative imagination and recalibrating your knowledgebase is kind of like being able to predict the future. In fact, this is exactly what engineers are paid to do! Predict how and when things will work or break!
Course structure:
Homework:
1) Worked problems assigned weekly, on Thursday, and due the next Friday (at 0800!).
2) Short reading quizzes delivered via D2L are also due weekly (Wednesday at 2400…or Thursday at 0000, depending on how you like to think about it).
Exams:
There will be three exams and a final exam.
Instructor expectations and availability:
I expect students to have read the relevant material in the course textbook prior to seeking help. Ideally, a student will have read prior to class. The textbook is the smartest resource in this course; instructors don’t have time to regurgitate everything in it during class nor should we have to during help sessions. Help sessions are for the especially difficult concepts that are still confusing even after reading it once or twice, thinking about it, sleeping on it, and then STILL not getting it.
Appointments are preferred and prioritized against random drop-ins.
Brushing Up:
Units of interest:
1) Energy! [kg·m2/s2, N·m, Pa·m3, Joule], Ft·lbs, BTU, Calorie, Kilocalorie, calorie, Ton of Refrigeration, kW·hr.
2) Also Kelvin, Celsius, Fahrenheit, Rankine.
3) Pascals, kilopascals, bars, pounds per square inch. Absolute vs. Gage?
Approximately how much raw thermal energy (heating value) is in the fuels we use?
|
Fuel |
Btu/lb |
kJ/g |
|
Diesel |
19,600 |
46 |
|
Gasoline |
19,500 |
45 |
|
Propane |
21,600 |
50 |
|
Liquefied Natural Gas (LNG) |
23,700 |
55 |
|
Liquefied Hydrogen (LH2) |
52,000 |
142 |
One Calorie equates to the amount of energy required to raise the temperature of one kilogram of water one degree Celsius.
How much work can a gallon of diesel do for us?
Assumption: A diesel engine can convert ~40% of the heating value of diesel into work, the rest is wasted.
1 gal Diesel ≈ 3.2 kg Diesel ⇒ ( 46 Mj / kg ) ( 3.2 kg ) = 147.2 Mj Heat ⇒ (0.40 ) ( 147.2 Mj ) ≈ 59 Mj work
This is equivalent to 43,516,730 ft∙lbs. If a loaded diesel pickup truck weights 10,000 lbs, this is enough energy to vertically lift it 4,352 ft.
How long would it take a person at peak energy output to lift the same pickup truck to the same altitude (assuming they had sufficient mechanical advantage via pulleys or gears or whatever)?
Assume an amazingly fit person can achieve 2 kW of work output and make them do it for as long as possible (by the way, this would certainly be fatal).
E = W Δt ⇒ Δt = E / E = 59,000 kj / 2 ( kJ / s ) = 29.5000 s = 8.19 hrs
59,000 kJ is equivalent to 14,100 Calories, which is 10 kilograms of cooked spaghetti. But how efficient is a human at converting Calories to work? Only about 25%. So, in order for this superperson to positively change the elevation of this 10,000 truck by 4,352 ft, she would need to consume 40 kilograms of cooked spaghetti (and, I imagine, even more water).
How much spaghetti would this person need to eat if her only job in life was to heat a room, itself far away from her, by using her useful work output to spin an electrical generator that heated the room via electrical resistance in an amount equivalent to that provided by just burning a gallon of diesel fuel? I don’t know, but I bet YOU could find out.
More unit stuff:
Power is energy per time. It can be “work per time” or it could be “thermal energy per time,” as in, “firepower” or “heat release rate.” Usually, though, when we use the term “power” in this class, it’s usually referring to mechanical or electrical work per unit of time (1 kW = 1 kJ/s, 1 HP (i.e. horsepower) = 550 ft∙lbs/s)
Kilowatt-hour is actually a unit of power (i.e. kilojoules per second) multiplied by a unit of time (1 hour) to yield an amount of energy. 1 kW∙hr = 3,600 kJ.
Pressure is the amount of force being exerted upon a unit of area, P = F/A.
If work is a force times applied over a distance, W = F∙Δx, then we can also say W = (P∙A)∙Δx = P∙(A∙Δx) = P∙(ΔV)
Pressure times a change in volume (usually of a gas) is equivalent to mechanical work! More on this later… Absolute pressure is the important one, but it’s not the one we usually talk about in everyday life.
Car tires are typically inflated to about 32 PSI Gauge, meaning the car tires are 32 PSI more than the local atmospheric pressure. In actuality, the inside of the tire is experiencing 32 + 14.7 = 46.7 psi of absolute pressure.
Pabsolute = Pgauge + Patmosphere
But, since that 14.7 PSI shows up both on the inside AND outside of the tire, we don’t really worry about it…UNLESS we’re doing thermodynamics homework and need to calculate, say, the mass of air inside the tire if we assume it’s at some absolute temperature.
Thermolinguistics:
Property: a characteristic of a system’s contents, such as pressure, temperature, volume, density, internal energy, etc.
Intensive properties: Don’t depend on mass for their value (i.e. pressure and temperature).
Extensive properties: Depend on how much (i.e. mass) of the substance exists in order for the value (Volume, Internal Energy, mass).
Specific properties: as we’ll use them, these are the quantity of an extensive property divided by how much mass is present (specific volume is m3/kg, or, “the amount of cubic meters taken up by one kilogram of substance).
State: a stable condition in which a system is everywhere homogenous and therefore in equilibrium with itself with a fixed set of properties throughout. For simple compressible systems, knowing a total of two intensive or specific properties fixes the state and allows to know ALL the other properties in that state.
Process: something that causes the system’s state (and its associated set of properties) to change to a different state with its own new set of properties. Compressing a gas, heating a glass of water, adding or removing mass, lifting to a higher elevation, accelerating or stopping a flowing fluid all constitute processes that change the state of the substance.
A collision process: (not actually a good example of the kinds of processes we’ll be studying)
State 1: Leftward translational velocity of F-4 Phantom = A Lot; State of aircraft = intact

State 2: Leftward translational velocity of F-4 Phantom = Still A Lot; State of aircraft = questionable

State 3: Leftward translational velocity of F-4 Phantom = Null; State of aircraft = What aircraft??

Source
Unknown Contributor - Reddit
Related:
- FIRST LAW OF THERMODYNAMICS
- SECOND LAW OF THERMODYNAMICS
- Thermodynamic Systems and Surroundings
- Types of Thermodynamic Systems
- Thermodynamic Equilibrium
- Control Volume
- Steady State
- Thermodynamic Process
- Cyclic Process
- Reversible Process
- Irreversible Process
- Adiabatic Process
- Isentropic Process
- Polytropic Process
- Throttling Process