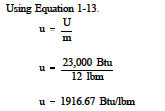Specific Internal Energy - Thermodynamic Properties
Thermodynamics Directory | Heat Transfer Directory
Specific Internal Energy
Potential energy and kinetic energy are macroscopic forms of energy. They can be visualized in terms of the position and the velocity of objects. In addition to these macroscopic forms of energy, a substance possesses several microscopic forms of energy. Microscopic forms of energy include those due to the rotation, vibration, translation, and interactions among the molecules of a substance. None of these forms of energy can be measured or evaluated directly, but techniques have been developed to evaluate the change in the total sum of all these microscopic forms of energy. These microscopic forms of energy are collectively called internal energy, customarily represented by the symbol U. In engineering applications, the unit of internal energy is the British thermal unit (Btu), which is also the unit of heat.
The specific internal energy (u) of a substance is its internal energy per unit mass. It equals the total internal energy (U) divided by the total mass (m).

Example:
Determine the specific internal energy of 12 lbm of steam if the total internal energy is 23,000 Btu.
Solution: