Triple Point - Thermodynamics
Thermodynamics Directory | Heat Transfer Directory
In thermodynamics , the triple point of a substance is the temperature and pressure at which the three phases ( gas , liquid , and solid ) of that substance coexist in thermodynamic equilibrium . For example, the triple point of mercury occurs at a temperature of −38.83440 °C and a pressure of 0.2 m Pa .
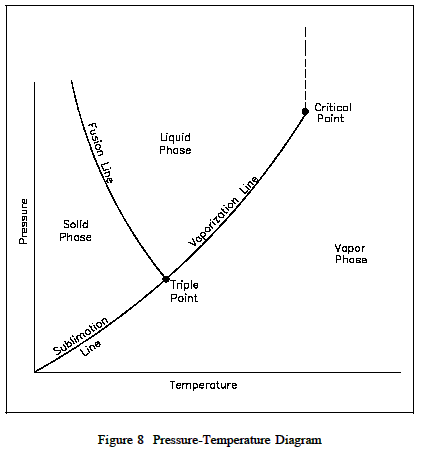
Finally, consider an initial pressure of the ice of 0.08854 psia. Again, as a result of heat transfer,the temperature will increase until it reaches 32F. At this point, however, further heat transfer may result in some of the ice becoming vapor and some becoming liquid because it is possible to have the three phases in equilibrium. This is called the triple point, defined as the state in which all three phases may be present in equilibrium. Figure 8 is a pressure-temperature diagram for water that shows how the solid, liquid, and vapor phases may exist together in equilibrium. Along the sublimation line, the solid and vapor phases are in equilibrium, along the fusion line, the solid and liquid phases are in equilibrium; and along the vaporization line, the liquid and vapor phases are in equilibrium. The only point at which all three phases may exist in equilibrium is the triple point. The temperature and pressure for the tri point because there is no distinct change from the liquid phase to the vapor phase above the critical point.